The actin cytoskeleton is composed of a plethora of single molecules, which are organized in filaments and superstructures thereof. The actin cytoskeleton is continuously being remodeled and this dynamic remodeling is essential for eukaryotic cells. It allows cells to respond to intracellular and extracellular stimuli and is indispensable for cell division, establishment and changes in cellular morphology and cell adhesion-processes. Such processes for example play an important role during embryonic development, wound healing as well as the formation of neuronal networks.
As cytoskeletal forces are a major source for defining, keeping and changing the morphology and subcellular organization of cells and these processes are crucial for the formation of the complex tissues and organs of multicellular organisms, we study the molecular mechanisms and the cellular functions of components of the actin cytoskeleton. We hereby focus on the machines that catalyze the critical step of actin filament formation, i.e. the assembly of an actin nucleus. These components are called actin nucleators.
The required morphological and functional organization of cellular membrane structures and compartments as well as of membrane transport processes depends on a coordinated work of a variety of molecules that are part of the membrane-associated, cortical actin cytoskeleton. One main research focus therefore is to identify and characterize molecular players at the functional interface between membrane dynamics and the cortical actin cytoskeleton.
Research Projects
The actin nucleator Cordon-Bleu (Cobl) - elucidation of regulatory mechanisms and cellular function
Actin filament polymerization is essential for processes as diverse as cell morphology control, the formation of multicellular networks, cell migration and membrane trafficking processes. Indispensable is thereby a tight spatial and temporal control of actin filament nucleation. With the multiple WH2 domain-containing protein Cobl, we have identified a novel and very powerful actin nucleator (Ahuja et al. 2007 Cell). Our studies have unraveled that Cobl plays an important role in proper formation of neuronal cell morphology - a prerequisite for the formation of functional neuronal networks (Ahuja et al. 2007 Cell; Schwintzer et al. 2011 EMBO J.; Hou et al. 2015 PLoS Biol.; Hou et al. 2018 Dev. Cell).
The successful establishment of Cobl-deficient organisms (Haag et al. 2018 Cell Rep.) has furthermore already allowed first insights into the role of this new actin nucleator in cellular development, differentiation and function in the intact organism. Among other phenotypes, these studies strikingly show that also different types of ciliary structures rely on the function of the actin nucleator Cobl.
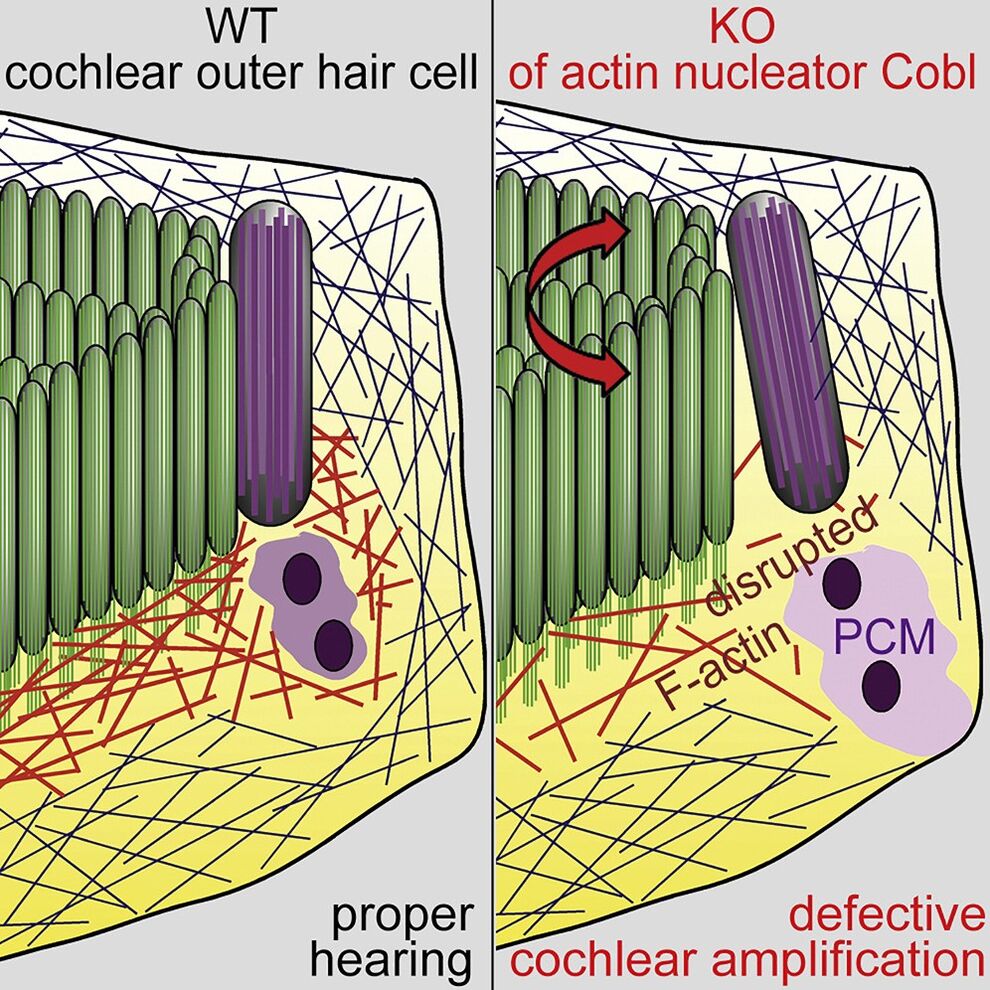
Proper cochlear hair cell array development and sensory apparatus positioning are achieved by planar cell polarity signaling. Our analyses show that the actin nucleator Cobl is an important effector in postnatal refinement and maintenance of planar cell polarity (Haag et al. 2018 Cell Rep.). During the critical time of hearing onset, these polarity defects coincided with reduced F-actin beneath the sensory apparatus and with premature kinocilium retraction. These defects were accompanied by organizational defects of the pericentriolar scaffold in Cobl KO mice that were actin polymerization-dependent and calcium/calmodulin signaling-dependent. The Cobl-dependent planar cell polarity maintenance and refinement processes we uncovered seem critical for hearing, as Cobl KO mice show deficient cochlear amplification (Haag et al. 2018 Cell Rep.).
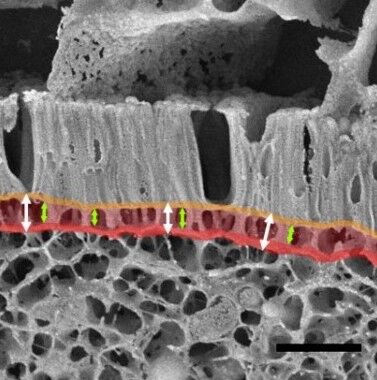
Our analyses furthermore demonstrated that the actin nucleator Cobl is critically involved in generating one of the cellular structures of the brush border-decorated apical cortex of enterocytes representing the absorptive intestinal surface. Quantitative analyses using both classical ultrastructural methods and a combination of deep-etching and high-resolution scanning electron microscopy of intact intestinal tissue samples provided unique insights into brush border organization and the crucial role of the actin nucleator Cobl (Beer et al. 2020 Sci Rep.)
Inhalt 1

Ischemic stroke is a major cause of death and long-term disability. Further studies of our lab demonstrated that induction of ischemic stroke in mice causes damages in dendritic arborization in peri-infarct areas, which then are repaired by processes of dendritic regrowth relying on the actin nucleator Cobl. In Cobl knockout mice, the dendritic repair window strikingly passed without any dendritic regrowth. Our results thereby highlight a powerful poststroke recovery process and identified causal molecular mechanisms critical during poststroke repair (Ji et al. 2021 PLoS Biol.).
Our successfully executed analyses of the consequences of a loss of Cobl at the cellular, tissue and organism level significantly expande our molecular understanding of the functions of the cortical actin cytoskeleton - functions that are indispensable for live of all higher eukaryots
Functional interdependence of the actin nucleator Cobl and Cobl-like in dendritic arbor development
Local actin filament formation is indispensable for development of the dendritic arbor of neurons. We show that, surprisingly, the action of single actin filament-promoting factors was insufficient for powering dendritogenesis. Instead, this required the actin nucleator Cobl and its only evolutionary distant ancestor Cobl-like acting interdependently (Izadi et al. 2021 eLife).
Interestingly, we also had discovered Cobl-like to be important for Ca2+/calmodulin-controlled neuromorphogenesis (Izadi et al. 2018 J. Cell Biol.). While Cobl uses three Wiskott-Aldrich syndrome protein Homology 2 (WH2) domains to nucleate actin (Ahuja et al. 2007 Cell), Cobl-like employs a unique combinatory mechanism of G-actin binding by its single, C terminal WH2 domain and Ca2+/calmodulin-promoted association with the actin-binding protein Abp1 to promote F-actin formation (Izadi et al. 2018 J. Cell Biol.).
The coordination between Cobl-like and Cobl was achieved by physical linkage by the membrane-binding F-BAR protein syndapin I, which we identified to interact with Cobl-like and to specifically occur in nanoclusters at the convex membrane surfaces at the base of nascent membrane protrusions of developing neurons (Izadi et al. 2021 eLife). Syndapin interacts with three motifs in Cobl-like, one of which was Ca2+/calmodulin-regulated. Consistently, syndapin I, Cobl-like's newly identified N terminal calmodulin-binding site and the single Ca2+/calmodulin-responsive syndapin-binding motif all were critical for Cobl-like's functions (Izadi et al. 2021 eLife).
In dendritic arbor development, local Ca2+/CaM-controlled actin dynamics thus relies on regulated and physically coordinated interactions of different F-actin formation-promoting factors and only together they have the power to bring about the sophisticated neuronal morphologies required for neuronal network formation in mammals.

Molecular mechanistic properties of novel N-Ank superfamily members, their functions and their interplay with BAR domain proteins in modulating membranes topologies and shaping cells
Shaping membranes is crucial for establishing, maintaining and changing the morphology of cellular organelles and of entire cells. Proteins that can directly power the required local membrane topology changes thus attract high interest.
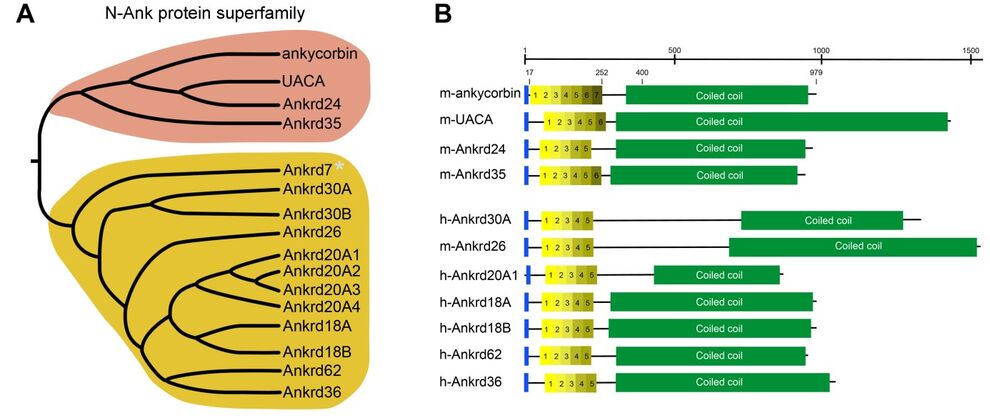
Ankyrin repeats adopt a curved structure and are one of the most frequently observed structural features in proteins. We recently proposed that, mainly based on the exemplary examination of ankycorbin, a newly discovered subset of ankyrin repeat proteins, which we termed N-Ank proteins, uses a combination of an amphipathic α-helix and ankyrin repeats to sense membrane curvature and to shape membranes (Wolf et al. 2019, Nat. Cell Biol.).
Inhalt 1
Our identification of an interaction of ankycorbin with another, distinct protein family of membrane shapers (Izadi et al. 2023 J. Cell Biol.) furthermore served as exciting starting point to examine whether and to what extent different membrane topology-recognizing and -modulating proteins cooperate in shaping membranes and entire cells. We address this novel concept by comprehensive biochemical analyses as well as by high-resolution imaging and functional studies in neurons (Izadi et al. 2023 J. Cell Biol.).
In summary, studying the mechanisms and the functional role of N-Ank superfamily members in neuromorphogenesis and examining their physical and functional interaction with membrane shapers of distinct nature will open an important new chapter in our understanding of the morphogenesis of cellular membranes and of entire cells.
Revealing lipid cues in cellular plasticity processes at ultra-high resolution
Not only neuromorphogenesis but also synaptic plasticity involves proper establishment and rearrangement of structural and functional microdomains. Applying a combination of rapid cryofixation, membrane freeze-fracturing, immunogold labeling and electron microscopy, our recent study provided ultrahigh-resolution views of the lipid PIP2 during long-term depression (LTD) induction.
We uncovered the spatial and temporal principles and the molecular mechanisms underlying PIP2 signals and their timely termination during LTD induction in subdomains of the dendritic spine membrane (Hofbrucker-MacKenzie et al. 2023 Comm. Biol.).
Further Research projects at the institute see:
- Institute for Biochemistry I - Britta Qualmann

