Our current studies aim at molecularly characterizing physiological and pathophysiological N-Ank proteins, a new class of powerful membrane shapers, and thereby assign mechanistic and cell biological functions to the N-Ank protein superfamily.

Characterization of physiological and pathophysiological N-Ank proteins in membrane shaping and cellular morphogenesis
Cells of multicellular organisms need to adopt specific morphologies. Most cells of multicellular organisms undergo sophisticated shape modulations during development. Neurons show extensive morphology changes during neuronal network formation. However, the molecular mechanisms bringing about membrane topology changes are far from understood – mainly because knowledge of membrane-shaping proteins that can promote local membrane curvatures is still limited (Kessels & Qualmann 2021, Curr. Opin. Cell Biol.). Since ankyrin repeats adopt a curved structure and several ankyrin repeat proteins additionally contain predicted coiled coil domains, we addressed the hypothesis that these proteins may be thus far unrecognized, self-assembling membrane curvature sensors or inducers. Indeed, we find that a subfamily of ankyrin repeat proteins, which we termed N-Ank proteins, uses a combination of an amphipathic α-helix and curvature sensing ankyrin repeats to shape membranes (Wolf et al. 2019, Nat. Cell Biol.). Characterizing ankycorbin as an example, we demonstrate that both of these molecular features of the N-Ank module are crucial for shaping local sites of membrane protrusion in primary hippocampal neurons during early neuromorphogenesis (Wolf et al. 2019, Nat. Cell Biol.). Crucial molecular mechanisms hereby are i) membrane insertion by an N terminal amphipathic α-helix, ii) curvature sensing by the curved array of ankyrin repeats and iii) coiled coil-mediated self-association to form ankycorbin-enriched nanodomains in the plasma membrane (Wolf et al. 2019, Nat. Cell Biol.). Our comparative characterizations of further N-Ank proteins – many of which interestingly seem to be human-specific and mostly uncharacterized – provide clear and comprehensive evidence that membrane shaping indeed is a common capability of the N-Ank modules of the new protein superfamily we identified (Wolf et al. 2019, Nat. Cell Biol.).
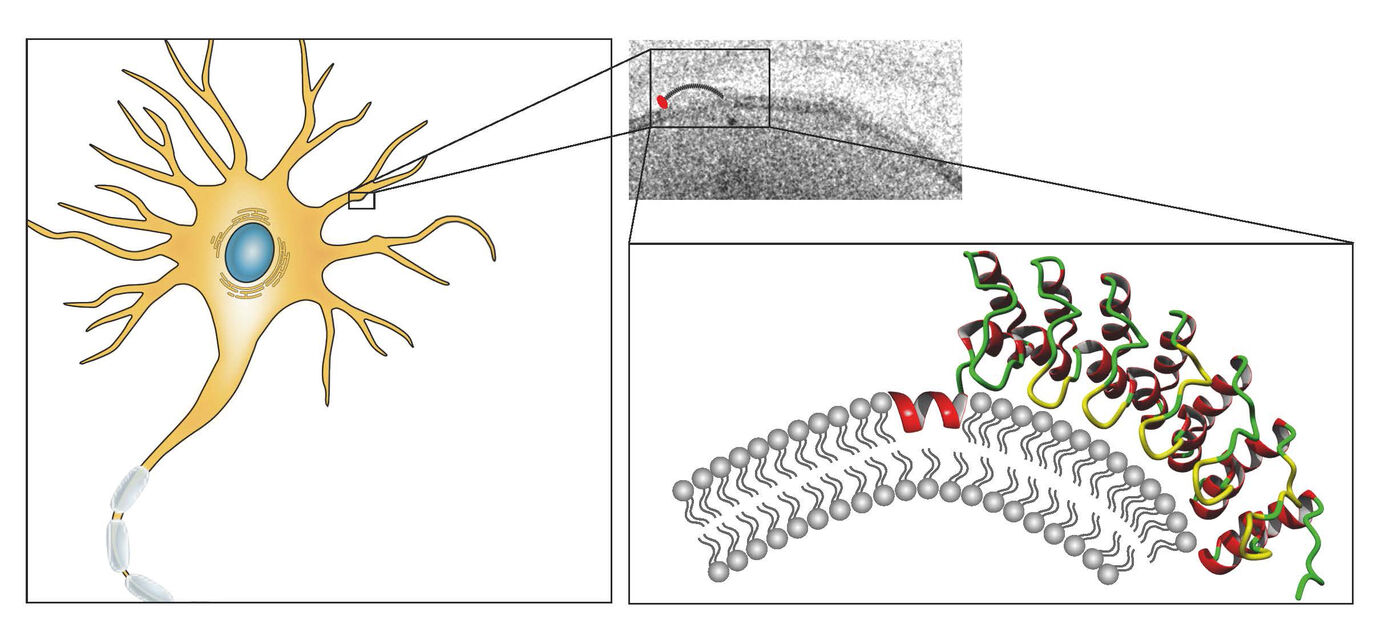
Our current studies aim at molecularly characterizing physiological and pathophysiological N-Ank proteins, a new class of powerful membrane shapers, and thereby assign mechanistic and cell biological functions to the N-Ank protein superfamily.
Neuronal morphology and network formation - Mechanism, control and cellular functions of Cobl-mediated actin nucleation
The complex architecture of neuronal networks and the extreme connectivity of cells within these networks is a basis for the function and abilities of vertebrate brains. Spatial and temporal control of cortical actin nucleation is indispensable for proper establishment and plasticity of cell morphology.
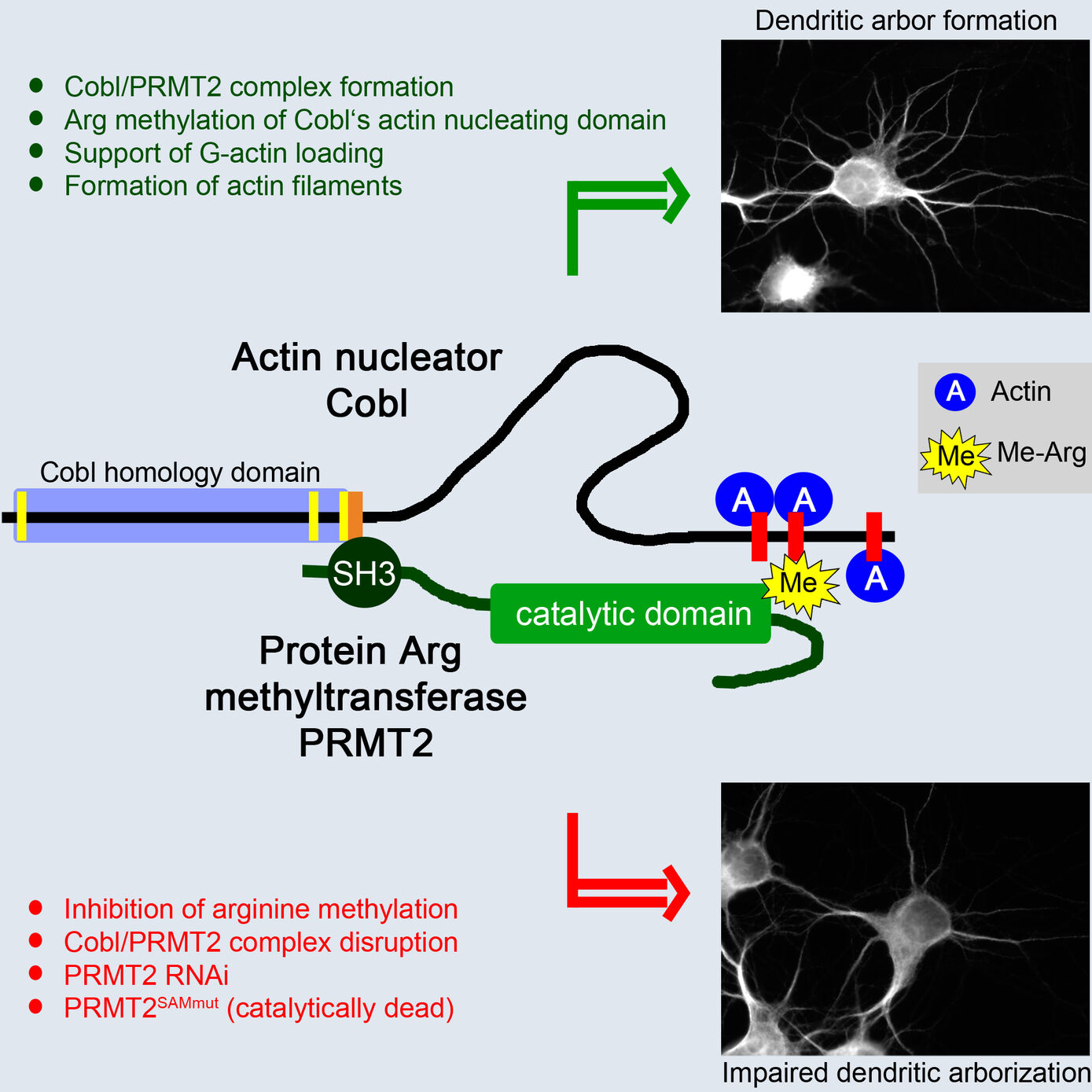
The formation of complex actin superstructures is indispensable for life of all eukaryotes. It critically relies on the help of factors facilitating the assembly of the first actin monomers, a process termed nucleation. With Cobl, we have identified a novel and very powerful actin nucleator indispensable for proper neuromorphogenesis (Ahuja et al. 2007, Cell). As excessive, uncoordinated actin nucleation would interfere with a huge variety of cellular processes relying on a proper organization of the actin cytoskeleton, it is obvious that the Cobl-mediated actin nucleation activity needs to be carefully regulated. The identification and functional characterization of Cobl protein interactions, which control the activity and/or the subcellular localization of this novel powerful machinery for actin nucleation, enables unravelling molecular mechanisms for interconnecting signaling processes to structural organization and plasticity in neurons (Schwintzer et al. 2011, EMBO J.; Hou et al. 2015, PLoS Biol.; Hou et al. 2018, Dev. Cell). Our identification of calmodulin, an important calcium sensor and signaling molecule, as an Ca2+-concentration-dependent interaction partner for Cobl unveiled a molecular mechanism, which interconnects neuronal calcium signaling to actin-driven neuronal arborization underlying neuronal network formation (Hou et al. 2015, PLoS Biol.).
The identification of Cobl as target for the arginine-protein-methyltransferase PRMT2 unveiled that, in addition to Ca2+/calmodulin signaling, posttranslational modifications control the potent actin nucleator Cobl (Hou et al. 2018, Dev. Cell). These studies will thus in particular significantly increase our molecular understanding of the actin cytoskeletal processes underlying neuromorphogenesis and the formation of neuronal networks.

Loss-of-function studies in whole organisms reveal crucial roles of F-BAR domain proteins in health and disease
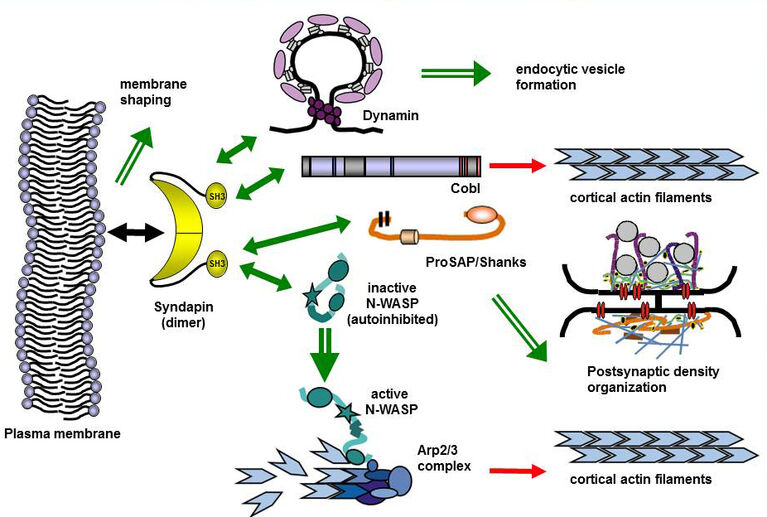
BAR superfamily proteins characterized by crescent-shaped membrane binding interfaces recognizing and inducing distinct curved membrane topologies have emerged as important components in shaping cellular membranes (Qualmann et al. 2011, EMBO J.; Kessels & Qualmann 2020, Biochem. Soc. Trans.; Kessels & Qualmann 2021, Curr. Opin. Cell Biol.). Our phenotypical analyses of knockout mice provide in vivo evidence for a physiological importance of the membrane-binding and topology-modulating F-BAR protein syndapin I in proper brain function. Syndapin I KO mice suffer from seizures, a phenotype consistent with excessive hippocampal network activity. Loss of syndapin I causes defects in presynaptic membrane trafficking processes, accumulation of endocytic intermediates, loss of synaptic vesicle size control, impaired activity-dependent synaptic vesicle retrieval and defective synaptic activity (Koch et al. 2011, EMBO J.). These phenotypical analyses of syndapin I KO mice thus highlight the functional importance of syndapin I for proper synaptic vesicle formation in hippocampal and retinal synapses, for proper hippocampal network activity and for proper function of the mammalian brain as such.
Our studies revealed that the F-BAR protein syndapin I is furthermore a crucial postsynaptic coordinator in formation of excitatory synapses (Schneider et al. 2014, J. Cell Biol.). Syndapin I deficiency caused significant reductions of synapse and dendritic spine densities. Ultrahigh-resolution imaging of specifically membrane-associated, endogenous syndapin I at membranes of freeze-fractured neurons revealed that membrane-bound syndapin I preferentially occurred in spines and formed clusters at distinct postsynaptic membrane subareas. Postsynaptic syndapin I deficiency led to defects in synaptic transmission (Schneider et al. 2014, J. Cell Biol.). Syndapin I–enriched membrane nanodomains thus seem to be important spatial cues and organizing platforms, shaping dendritic membrane areas into synaptic compartments.
Schizophrenia is associated with cognitive and behavioral dysfunctions thought to reflect imbalances in neurotransmission systems. Syndapin I KO mice developed schizophrenia-related behaviors, such as hyperactivity, reduced anxiety, reduced response to social novelty and an exaggerated novel object response and exhibited defects in dendritic arborization in the cortex. Neuromorphogenic deficits were also observed for a schizophrenia-associated syndapin I mutant in cultured neurons and coincided with a lack of syndapin I-mediated membrane recruitment of cytoskeletal effectors (Koch et al. 2020, Cereb. Cortex). Syndapin I KO furthermore caused glutamatergic hypofunctions. Syndapin I regulated both AMPA receptor and NMDA receptor availability at synapses during basal synaptic activity and during synaptic plasticity – particularly striking was a complete lack of long-term potentiation and defects in long-term depression in syndapin I KO mice. These synaptic plasticity defects coincided with alterations of postsynaptic actin dynamics, synaptic AMPA receptor clustering, mobility and internalization (Koch et al. 2020, Cereb. Cortex). Thus, syndapin I KO led to schizophrenia-like behavior and our analyses uncovered associated molecular and cellular mechanisms.
Interestingly, our analyses furthermore revealed an additional crucial role of syndapin I in inhibitory neurotransmitter receptor organization (del Pino et al. 2014, J. Biol. Chem.; Langlhofer et al. 2020, J. Neurosci.).
In summary, our investigations on different levels ranging from molecules to whole organisms have led to a deeper understanding of pre- and postsynaptic organization and membrane trafficking processes as well as the function of syndapin I therein and have furthermore provided important new insights into the molecular mechanisms of neurotransmission and synaptic plasticity including their physiological relevance.
The role of membrane-shaping BAR domain proteins in caveolar invagination: From mechanistic insights to pathophysiological consequences
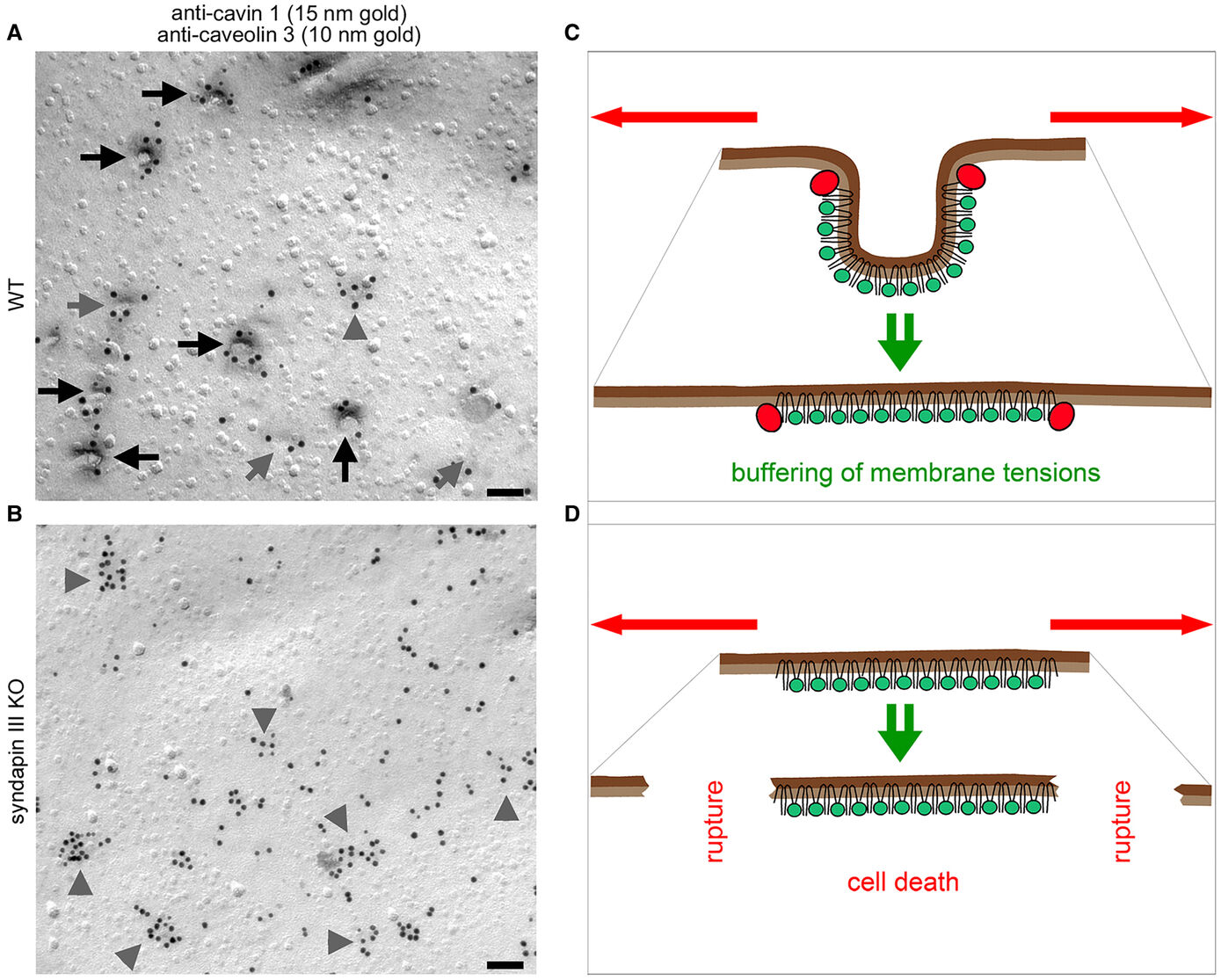
The formation of caveolae, bulb-shaped plasma membrane invaginations, requires the coordinated action of distinct lipid-interacting and –shaping proteins. The interdependence of the structure of caveolar invaginations with their function has evoked substantial scientific interest considering the association of several human diseases with caveolar dysfunction. Model systems deficient of core components of caveolae did not allow for an explicit attribution of observed functional defects to the requirement of caveolar invagination as they lack both invaginated caveolae and caveolin proteins (Kessels & Qualmann, 2020, Biochem. Soc. Trans.) Our studies revealed that syndapin III loss-of-function resulted exclusively in impairment of caveolar invagination not accompanied by any reduction of caveolin or cavin at the plasma membrane allowing for specifically unveiling the functions of caveolar invaginations as such (Seemann et al. 2017, Elife).
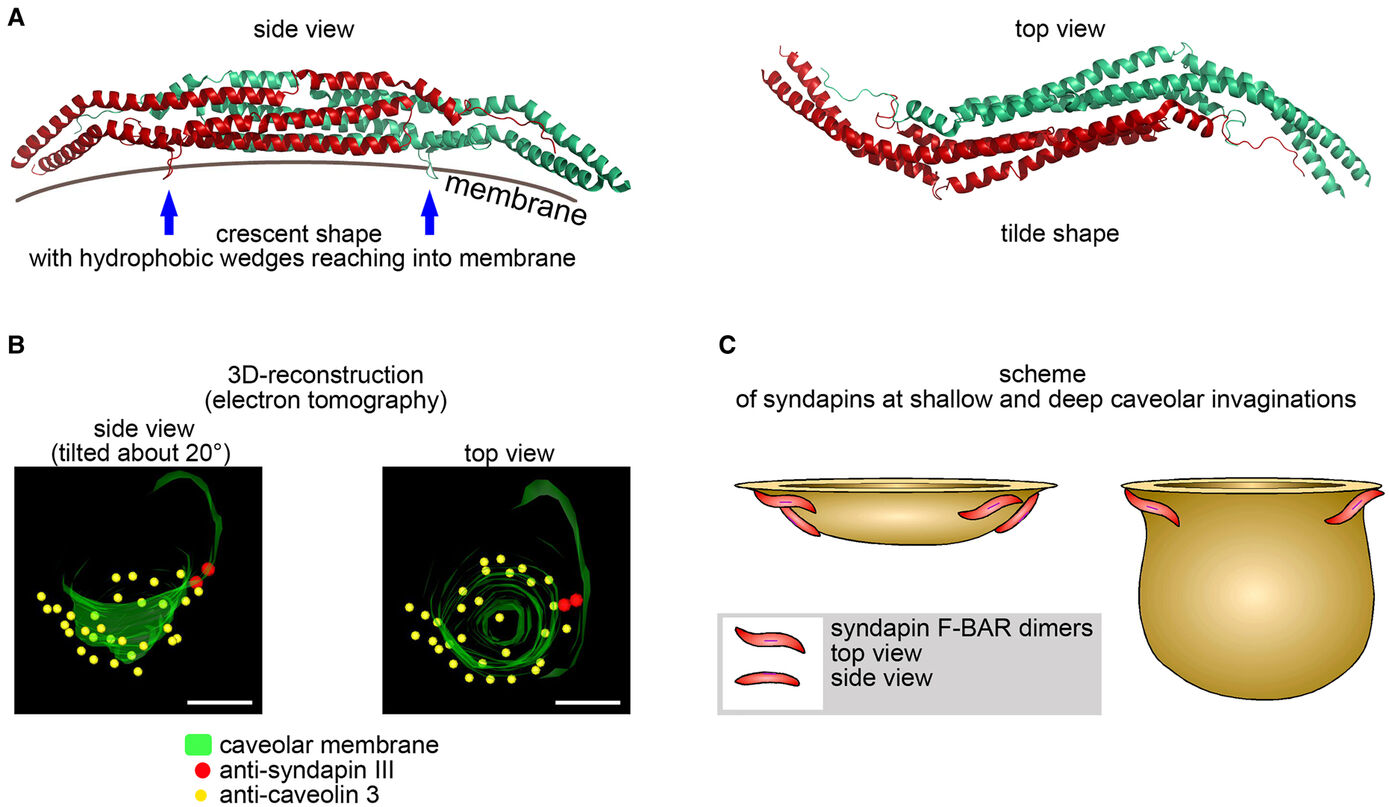
Muscle cells of syndapin III KO mice showed severe reductions of caveolae reminiscent of human caveolinopathies and were more vulnerable to membrane damage upon changes in membrane tensions in line with a function of caveolae in mechanoprotection by providing membrane reservoirs available through caveolar flattening. Consistently, upon physical exercise, failure to invaginate muscular caveolae using the membrane-shaping syndapin III resulted in pathological defects reminiscent of the clinical symptoms of human myopathies suggesting that the ability of muscular caveolae to respond to mechanical forces is a key physiological process (Seemann et al. 2017, Elife).
(for detailed information also see our publications and our research reports )
Further Research projects at the institute see:
