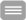Numerous studies have shown that inappropriate antimicrobial treatment, e.g. due to antimicrobial resistance, underdosing, and / or delayed initiation, dramatically increases mortality in sepsis. Mounting clinical evidence indicates that up to 80% of patients suffering from severe sepsis/septic shock exhibit subinhibitory antibiotic plasma concentrations using conventional fixed doses, mainly due to gross physiological derangements. This can lead to treatment failure and selection of resistant organisms. Given the paucity of new antimicrobial agents, optimized use of available antibiotics is indispensable to improve clinical outcome, particularly in the era of increasing resistance. Therapeutic Drug Monitoring (TDM) will be the topic of initially two clinical studies and a translational project, aiming to achieve predefined PK/PD targets by implementing TDM in patients with severe sepsis/septic shock and in patients with hematological malignancies and febrile neutropenia. The second topic of this research area and aim of a study is the early diagnosis of invasive Candida infections in patients at risk.
The core of this research area is formed by three clinical trials and one translational project:
C
CandiSepProspective randomized study of (1,3)-β-D-glucan guided diagnosis of invasive Candida infection versus culture-based diagnosis in patients with severe sepsis or septic shock and high risk of invasive Candida infections
Not only bacteria, but also infections of blood and abdominal cavity by candida (yeasts) can cause severe sepsis. Due to acquired weakness of the immune system and the necessary use of broad spectrum antibiotics intensive care unit patients are especially susceptible to invasive candida infections. Mortality is high. Detection of candida from blood by classic microbiological testing is only successful in half of all cases and takes several days. But already a delay of one day in the initiation of treatment with activity against candida results in a significantly higher mortality.
Therefore alternative strategies for the detection of candida infections are necessary. (1,3)-b-D-Glucan (BDG) is an important cell wall component of candida, but also of other fungal species. An assay (FungitellÒ) for its detection from blood is available. Available studies show that this test is positive in about 80% of critically ill patients with invasive candida infections, mostly several days before successful microbiological detection. A relatively high rate of false positive results is, however, problematic. Studies addressing the question whether its use in critically ill patients can improve treatment and outcome are lacking.
We want to close this gap in evidence with a multicentric clinical trial. We want to include 368 critically ill patients with new onset severe sepsis and risk factors for invasive candida infections. In addition to microbiological tests BDG will be measured. The result will be reported to the treating clinicians in the intervention group only, where it can result in early initiation of adequate therapy. In the control group the test result will not be available for the planning of therapy. We will evaluate whether the inclusion of BDG measurement can reduce 28-day mortality in risk groups.
S
SmartdoseSimultaneous determination of multiple antibiotics by LC-MS/MS in plasma, tissue, and breath condensate for personalized antibiotic treatment
Adequate antibiotic treatment is the prerequisite for successful treatment of systemic infections. Increased scientific evidence shows that a fixed dosage regimen can lead to insufficient and ineffective antibiotic therapy. This is caused not only by the development of antibiotic resistance, but also by a changed pharmacokinetics (PK) in critically ill patients. To personalize the therapy a quantification of the plasma concentration of antibiotics by Therapeutic Drug Monitoring (TDM) can be applied.
The goal of the project Smartdose is to develop an LC-MS/MS-based method allowing simultaneous quantification of different antibiotics in serum or plasma. In addition, the newly developed method will be applied also to tissue samples and respiratory gas condensates in order to investigate whether the antibiotic concentration in plasma is associated with a sufficient amount at the site of action.
A TDM of piperacillin/tazobactam concentrations is the first application of the LC-MS/MS method, performed in a collaboration with the clinical trials Target and Target-FN. In addition, the establishment and validation of alternative methods (e.g. Raman spectroscopy) is planned in selected materials.

Figure: Example chromatogram for anti-infectives
The peaks are shown in an overall overview of positive and negative ionization, including retention times in a sample chromatogram for 1 mg/l of anti-infective agent in EDTA plasma. Left: Chromatogram of all analytes with positive ionization, right: Chromatogram of all analytes with negative ionization. (Master thesis Christina Wichmann 2016: Simultaneous quantification of clinically relevant anti-infectives in blood plasma using LC-MS / MS)
T
TargetTherapeutic Drug Monitoring for Piperacillin/Tazobactam
Administration of antibiotics active against the infecting organism is a cornerstone of effective sepsis management. Management is however complicated by increasing antimicrobial resistance with shortage of new antimicrobial substances. Given the paucity of new antibacterial agents to optimize antimicrobial use and bring out the best of the currently available agents is indispensable. Increasing evidence indicates however that antibiotic dosing in critically ill patients is inadequate with fixed-dose regimens. Pharmacokinetic (PK) and pharmacodynamic (PD) studies during pharmaceutical registration trials are usually conducted in healthy, normal weighted young adults in most instances. However, critically ill patients have a gross physiological derangement, which profoundly impacts PK. Patients with sepsis are known to become hyperdynamic, causing increased clearance of ß-lactams; end-organ dysfunction can lead to impaired drug clearances and capillary leak syndrome results in increased interstitial fluid volumes. Inadequate concentrations, however, negatively affect infection-related patient outcomes and promote the development of antibiotic resistance. Methods to optimize administration of ß-lactam antibiotics in these patients are urgently required. Hence, the primary study goal is to investigate whether a therapeutic drug monitoring (TDM) - based dose optimization of piperacillin positively affects outcome in patients with severe sepsis or septic shock.
Therapeutic drug monitoring (TDM) for personalized antibiotic treatment with piperacillin-tazobactam (PipTaz) in patients with febrile neutropenia
Febrile neutropenia (FN) occurs in 80% of patients with myelosuppressive chemotherapy and is usually the first sign of a systemic infection, the leading cause of treatment-related mortality in cancer patients. Most episodes are assumed to be due to bacterial infections in this immunosuppressed patient population. Thus, febrile neutropenia is always treated empirically with broad-spectrum antibiotics. The most widely used antibiotic agent in this setting is piperacillin-tazobactam (PipTaz), which leads to resolution of fever in approximately 50% of cases. The remaining 50% of patients with FN require salvage treatment with antibiotics of the last resort, leading to increased toxicity and high risk of multi-drug resistance. Preliminary data indicate that subtherapeutic drug levels may in part be responsible for therapy failure.
The clinical trial Target-FN assesses feasibility and efficacy of personalized application of PipTaz using therapeutic drug-monitoring (TDM) in patients with hematological malignancies and FN. The study aims to raise the response rate from 50% following standard therapy to 70% using TDM. Target-FN is a prospective, monocentric, randomized clinical trial. PipTaz is administered in conventional dosage to the control group and TDM samples are used for diagnostic evaluation only. In contrast, PipTaz dosage in the intervention group is adjusted according to TDM results.
The response rate is recorded as stable defervescence, i.e. 5 consecutive afebrile days. In addition, length of treatment and of hospital stay as well as survival is recorded. Target-FN is the first clinical trial on TDM in FN. If successful, a direct impact on clinical practice and guidelines is foreseeable.