Scrutinizing the function of the cyclic-nucleotide binding domain in the activation of HCN channels
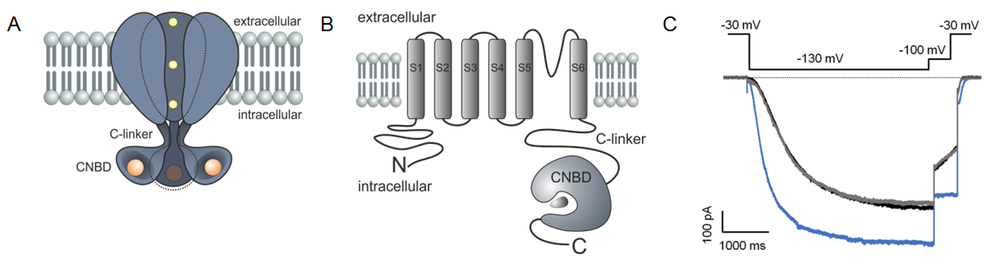
In the proposed project we intend to study the function of the C-linker and the cyclic-nucleotide binding domain (CNBD) in mammalian HCN (hyperpolarization-activated and cyclic-nucleotide modulated) channels and in homologue bacterial SthK channels of Spirochaeta thermophila. Both channel types are composed of four subunits, with the C-terminus of each subunit containing a cyclic nucleotide binding domain (CNBD) that is linked to the last transmembrane helix (S6) via the C-linker (CL). Patch-clamp fluorometry (PCF), a technique which combines electrophysiological approaches and fluorescence microscopy will be applied in two variations:
1) to monitor binding of the fluorescent cAMP derivative fcAMP as function of the activation state and
2) to analyze activation-induced conformational changes of the CL and CNBD employing either the intrinsically fluorescent unnatural amino acid Anap or Förster Resonance Energy Transfer after incorporating an appropriate donor and acceptor.
The data will be interpreted kinetically by Markovian models and by MD simulations in collaboration with H. Gohlke (P7). F. Noé and N. Plattner (P8) will provide novel strategies to fit Markovian models to the data.
Prof. Dr. Klaus Benndorf
Kollegiengasse 9
07743 Jena
Phone: +49 3641 9397651
E-mail:
Dr. Jana Kusch
Kollegiengasse 9
07743 Jena

