Dynamics of permeation and activation of AMPA receptors
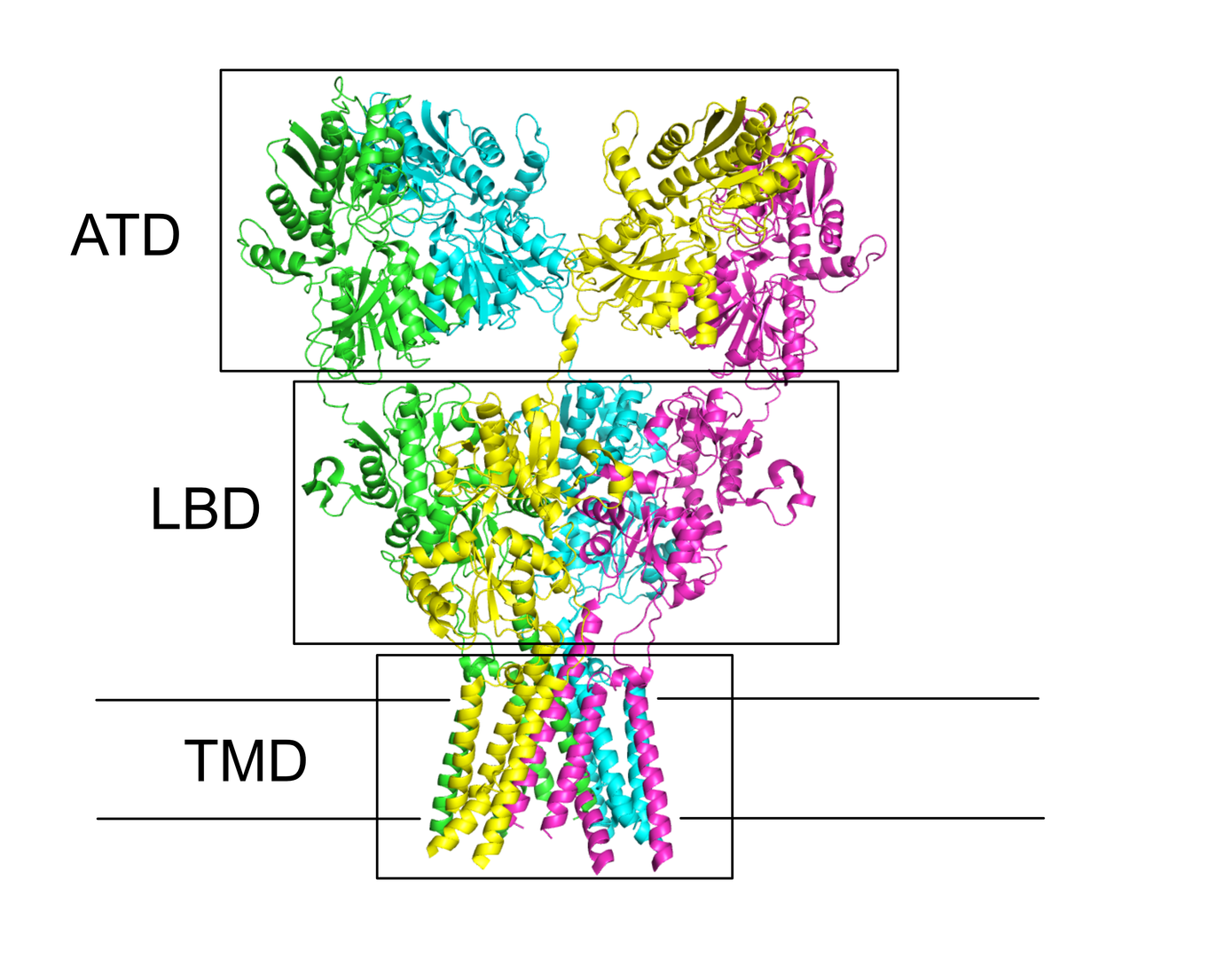
The activation of AMPA-type glutamate receptors at excitatory synapses is a fundamental event in the nervous system, essential for complex thought. The molecular mechanisms govern cation permeability in AMPA receptors (including that of the critical second messenger Ca2+) are opaque, because all structures to date have a closed pore and lack ordered selectivity filters. We aim to crystallize congeners of the GluA2 pore using prokaryotic ion channels as scaffolds. We will validate the suitability of candidates for all-atom simulations of ion permeation with electrophysiology. Armed with structural models we will simulate cation permeability according to various pore chemistries germane to native channels. We will test the ensuing predictions with single channel recording. AMPA receptor complexes are decorated with numerous auxiliary proteins that act as chaperones but also endow receptors with unusual gating properties, through largely unknown mechanisms. In a second, independent approach, we propose to identify structural mechanisms that underlie auxiliary protein action. We will co-crystallize glutamate receptor domains with segments of auxiliary proteins and build Markov state models of the dynamics of the key functional unit of AMPA-receptor activation, the active dimer of ligand binding domains. The influence of interactions with auxiliary proteins on these dynamics will be assessed from short, off-equilibrium atomistic simulations, and we will perform electrophysiological experiments on mutants derived from these results.
Prof. Dr. Andrew Plested
Humboldt-Universität zu Berlin
Lebenswissenschaftliche Fakultät
Institut für Biologie
Invalidenstraße 110
10115 Berlin
Dr. Han Sun
13125 Berlin

