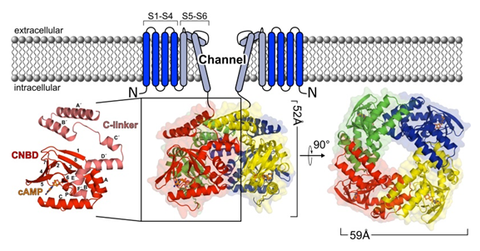
Disinhibition and inhibition of HCN2 channel function by ligand binding to the cyclic nucleotide binding domain
Hyperpolarization activated cyclic nucleotide modulated (HCN) channels are relevant disease factors, are activated by cAMP and cGMP, and consist of four subunits. Modulation of the channel function is conferred by the C-terminal region containing a cyclic nucleotide binding domain (CNBD) and a C-linker (CL) region. We address the central question how changes in the conformational dynamics and energetics of the tetrameric CL-CNBD of HCN2 upon cyclic nucleotide binding relate to the ligand-dependent channel gating. We intend to answer this question at the atomistic level by molecular dynamics simulations, alchemical and configurational free energy calculations, and rigidity analyses, in close connection with experimental data from project P2, which aims at experimentally resolving conformational changes of the CL-CNBD upon stepwise cyclic nucleotide binding. Establishing a successful combination of adequate computational approaches for model building and hypothesis generation and high-content experimental platforms for validation and providing feedback could become paradigmatic for investigating similarly complex ion channels.