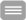Scrutinizing the function of the cyclic-nucleotide binding domain in the activation of HCN channels
In this project we study the ligand-dependent gating of cyclic nucleotide (CN)-modulated ion channels, thereby focusing on mammalian HCN (hyperpolarization-activated cyclic-nucleotide modulated) channels. HCN channels are tetramers, in which CN-induced gating is mediated by four intracellular C-terminal binding domains (CNBDs), linked to the last transmembrane helices (S6) via C-linkers (CLs), thereby also forming a tetrameric structure.
Conformational changes in this intracellular channel portion are studied by electrophysiology, mutagenesis and patch-clamp fluorometry, combining electrophysiology and fluorescence microscopy. Besides studying the kinetics of ion currents and fluorescence changes of environment-sensitive dyes, caused by conformational changes of the CL and CNBD, the binding of fluorescently tagged cAMP derivatives will be monitored. The data will be interpreted kinetically by MD simulations in collaboration with H. Gohlke (P7) and by extending our Markovian modeling strategies, thereby collaborating with F. Noé (P8).
Prof. Dr. Klaus Benndorf
Kollegiengasse 9
07743 Jena
Phone: +49 3641 9397651
E-mail:
Dr. Jana Kusch
Kollegiengasse 9
07743 Jena