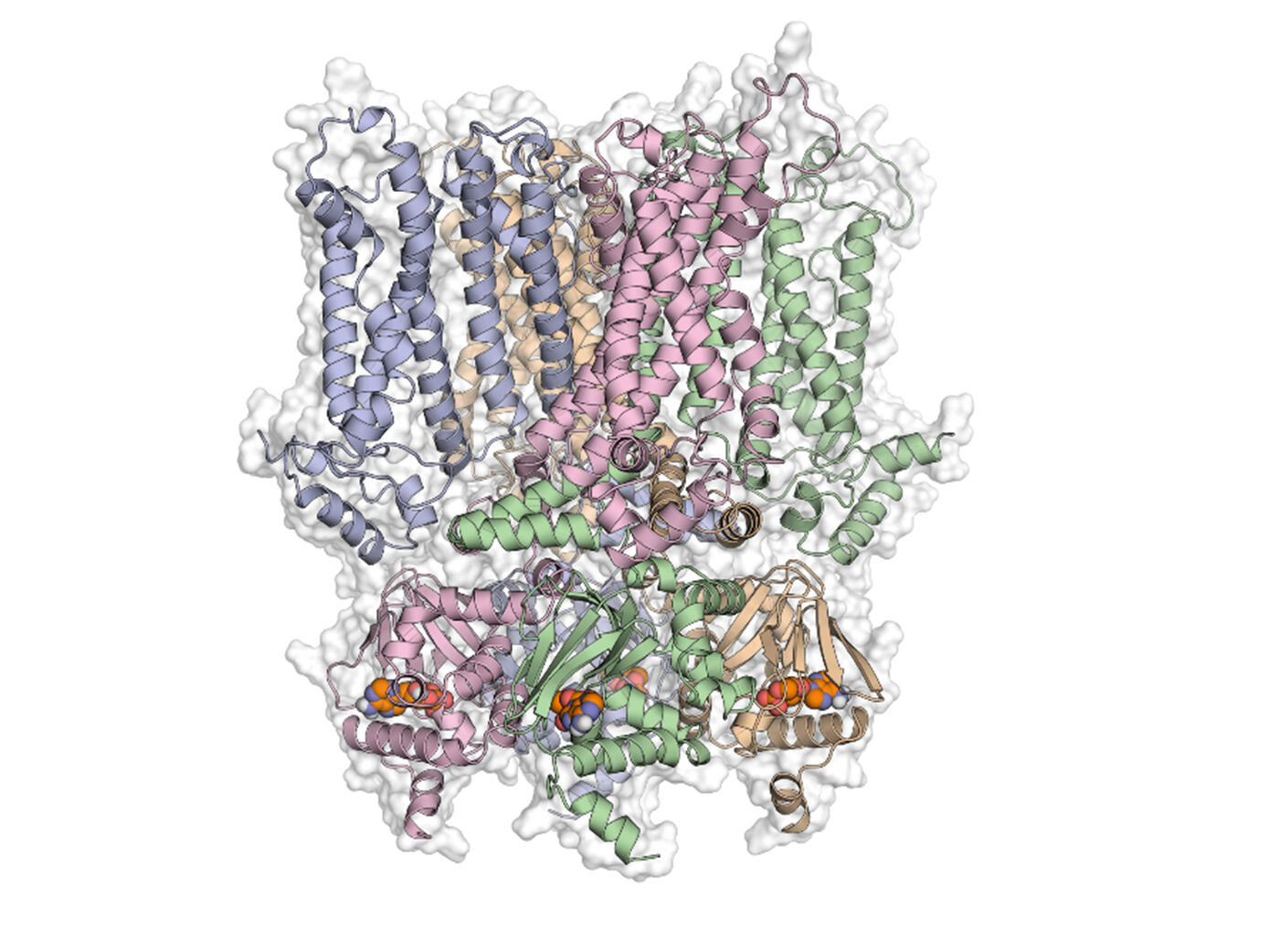
Disinhibition and inhibition of HCN2 channel function by ligand binding to the cyclic nucleotide binding domain
Hyperpolarization activated cyclic nucleotide modulated (HCN) channels are relevant disease factors, are activated by cAMP and cGMP, and consist of four subunits. Modulation of the channel function is conferred by the C-terminal region containing a cyclic nucleotide binding domain (CNBD) and a C‑linker (CL) region. We address the central question how changes in the conformational dynamics and energetics of the tetrameric CL-CNBD upon cyclic nucleotide (cNMP) binding relate to the ligand‑dependent channel gating, focusing on mammalian HCN2. We intend to answer this question at the atomistic level by molecular simulations and modelling, in close connection with experimental data from project P2, exploiting those full‑length structures of hHCN1 and hHCN4 have recently become available.
In the first funding period, we I) characterized novel cAMP and cGMP derivatives substituted at N8 by either hydrophobic alkyl chains or similar-sized more hydrophilic heteroalkyl chains and suggested an intricate enthalpy - entropy compensation underlying the higher apparent affinity of the derivatives with the longer alkyl chains, II) tailored a series of novel fluorescent cAMP or cGMP derivatives by attaching dyes via alkyl linkers to N8, III) demonstrated that N6‑modified cAMP derivatives that activate protein kinase A also act as full agonists of murine HCN2 channels, IV) developed an ensemble- and rigidity theory-based perturbation approach to analyse dynamic allostery and V) applied it to a membrane-bound transporter, receptor, and HCN2. As to the latter, predicted pathways are indicative of the influence of altered structural dynamics on allosteric signalling among four CL-CNBD subunits and, thus, provide a first atomistic interpretation of intersubunit cooperativity in HCN2 channels. In further, preliminary work, we VI) functionally and structurally characterized interactions between opposing subunits in HCN2 and VII) probed the impact of uncoupling the CL-CNBD from the transmembrane core by glycine insertions.
We these results at hand, in the next funding period, we will scrutinize I) how interactions between opposing subunits impact activation-dependent CL-CNBD rotation as well as II) allosteric coupling between the “opposing subunit region” and the cNMP binding site, III) investigate signal transmission between the CL-CNBD and the transmembrane core in variants with a defined number of uncoupled and/or functional CL-CNBDs, IV) probe the role of helices D and E on cAMP affinity and allosteric signal transmission, V) develop selective HCN agonists and identify inhibitors that stabilize the inactive state of the CL-CNBD, and, if time permits, VI) generate a structural model of HCN2 with dilated channel structure. These studies will significantly advance our understanding of how structural dynamics at the atomistic level and disinhibition and inhibition of HCN2 channel function are coupled by ligand binding to the CL-CNBD.