Patch-Clamp Fluorometry
The patch-clamp technique allows to measure precisely the currents flowing through ion channels. However, the power of electrophysiological techniques for revealing the mechanism of ion channel gating is limited, since only the last step of a sequence of conformational changes leading to the opening of a channel is accessible to elctrophysiological measurements. As long as no charge is translocated in the electrical field of the membrane, conformational rearrangements are electrically silent and cannot be detected by patch-clamp measurements.
This gap can be closed by optical techniques, which allow to monitor the binding of fluorescently labelled ligands to receptor channels or to trace conformational changes of labelled channel subunits.
Studying ligand binding allows to trace the first steps in the gating of ligand-gated channels. By measuring the fluorescence intensities of bound ligands the dose-binding relationship can be obtained, whereas electrophysiological measurements yield the dose-activation relationship. These data sets allow to test, verify (or falsify) different models of channel activation. An example for simultaneous measurements of ligand binding and channel activation of cyclic nucleotide gated (CNG) channels is shown in Fig. 1 and Fig. 2.
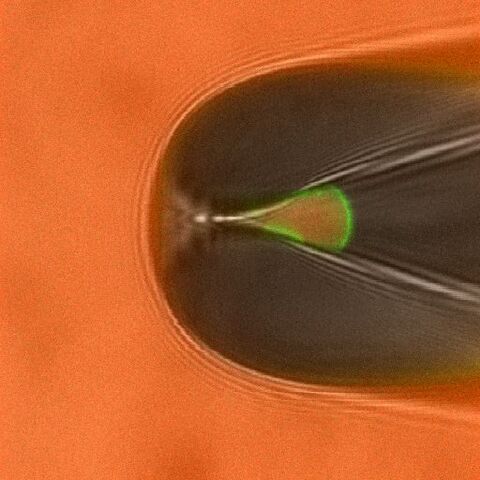
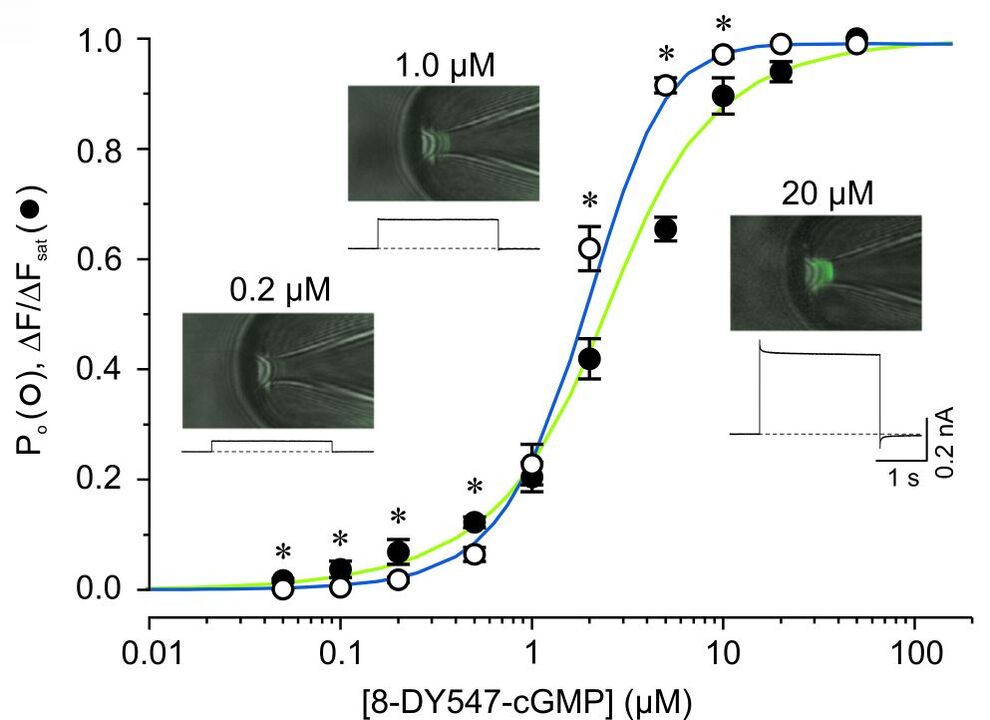
By attaching appropriate donor and acceptor fluorophores to segments involved in channel gating and static parts of the channel or the plasma membrane the relative movement can be traced via Förster resonance energy transfer measurements. These strategies are now tested in current projects.

