Cyclic nucleotide-gated (CNG) channels play an important role in the visual and olfactory system. They constitute the final step of a signal transduction cascade by which light or scent are transduced into a change of the membrane potential. Native CNG channels are heterotetramers, consisting of up to three different subunits. Each subunit has six membrane-spanning segments. The binding of a cyclic nucleotide (cAMP or cGMP) to the cyclic nucleotide binding domains (CNBDs) located intracellularly at the C-terminus promotes an allosteric conformational change that is transmitted through the C-linker to the channel pore leading to channel opening.
Although all subunits contain an intracellular located cyclic nucleotide-binding domain, only the CNGA1, CNGA2 and CNGA3 are capable of forming functional homotetrameric channels when expressed in heterologous expression systems. CNGA4 and CNGB subunits only modulate channel function and control plasma membrane targeting. Direct evidence for their contribution to channel activation by ligand binding was presented up to now only for the olfactory CNGA4 subunit.
This project aims at elucidating the role of the CNGB1a and CNGB3 subunits of our visual system. Specifically, we want to find out whether these subunits bind cGMP at physiological concentrations and if so, in which sequence binding to the respective subunits occurs.
The following questions will be addressed: In which sequence do the ligands bind to the subunits? Is the opening of the channel pore the result of a concerted conformational change of all four subunits? What conformational changes are induced by ligand binding? To address these questions we will use among other methods confocal patch-clamp fluorometry (cPCF) and Förster Resonance Energy Transfer (FRET) measurements.
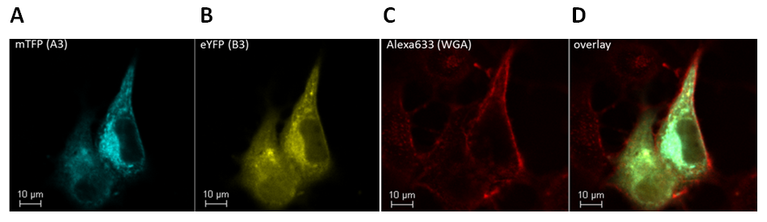
A second focus of the project will be on the mechanisms by which the cell ensures that channels are expressed in a fixed stoichiometry. These mechanisms are responsible for the successful channel trafficking to the plasma membrane whose malfunction was shown to be the cause of many channelopathies. By using fluorescence lifetime based FRET measurements we will investigate in which compartment the channels assemble, which subunits associate first, and how channel transport is controlled.
Dr. Vasilica Nache
Phone: 03641 - 9 397668