
Center for Sepsis Control and Care,
Center for Molecular Biomedicine
Lipids are not only present in cells to enable the assembly of membranes, organelles, and other structural components, but are also known as signaling molecules outside and within cells. The lipid signaling molecule sphingosine 1-phosphate (S1P) for example is a potent immune modulator that regulates proinflammatory cytokine secretion, vascular integrity, antigen presentation, and lymphocyte circulation. It is systemically produced in blood and lymph by red blood cells (RBC) and endothelial cells (EC) expressing sphingosine kinases (SphK) type 1 and 2, and it is irreversibly degraded by the S1P-lyase in tissue cells to ensure low S1P-levels in peripheral organs. The multitude of cellular responses to systemic and local S1P stimuli via five G protein-coupled cell surface receptors and diverse intracellular targets make S1P an interesting target molecule for similarly complex immune-associated diseases like sepsis.
Sepsis is a systemic disease and affects many different cell types, organs, and bodily functions. Understanding the various signaling processes within and between cells is indispensable in order to find new diagnostic tools and treatment options. The lipid signaling network is characterized by a high heterogeneity of lipid-specific G protein-coupled cell surface receptors, their expression pattern, local and systemic presence of lipid signaling molecules, and the dynamics of lipid metabolism. Major challenges are to unfold specific lipid signaling pathways and to detect and quantify lipids in biological samples. This course will offer experiments focusing either on the detection and quantification of various lipids and other metabolites using liquid chromatography coupled to triple-quadrupole mass spectrometry (LC-MS/MS) or on the signaling capabilities and pathways induced by lipid signaling molecules like S1P using various approaches including intracellular calcium flux measurements, flow cytometry, quantitative PCR, Western blots, enzyme-linked immunosorbent assays (ELISA) and fluorescent microscopy.
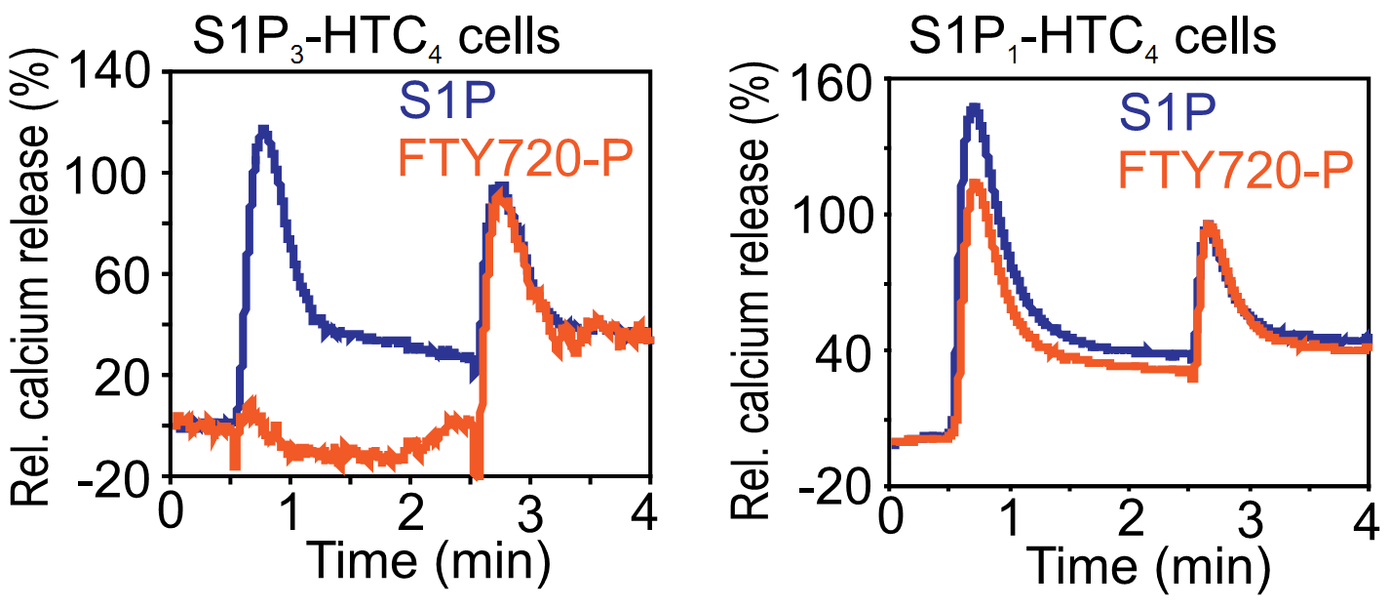
Fig. 1: Intracellular calcium trace of the rat hepatoma cell line HTC4 and HTC4 cells expressing S1P1 or S1P3 receptors. Cells were stimulated after 0.5 min with 100 nM S1P (blue) or 1 μM of the S1P-analog FTY-P (red). Stimulation with 10 μM ATP after 2.5 min was used for normalization (=100%).
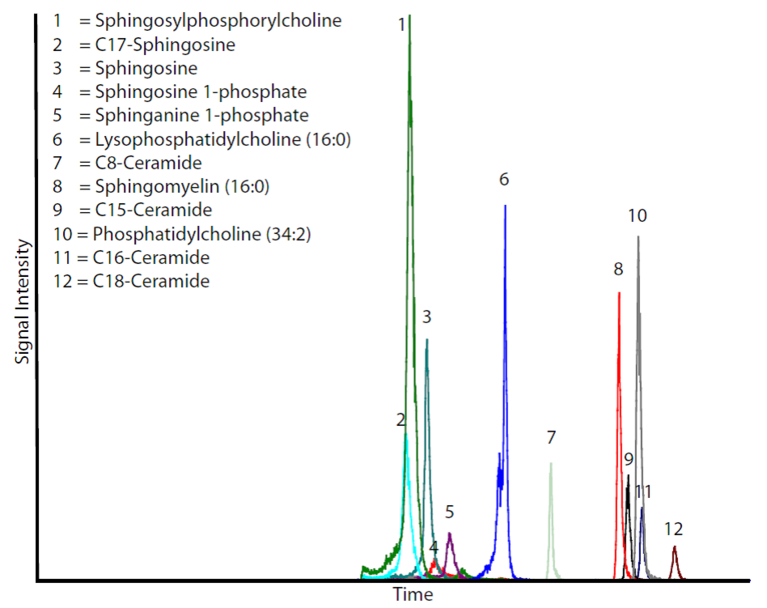
Fig. 2: Typical histogram of an LC-MS/MS analysis of 30 pmol of various lipids.


