

An important feature of various cell types, such as neurones, myocytes or cardiac cells, is their excitability, a key function for fulfilling their specific tasks. Being excitable is achieved by expressing ion channel proteins, allowing ion fluxes across the cell membrane. The activity of these channel proteins can be controlled by different stimuli. In the case of ligand-gated ion channels, also called ionotropic receptors, the controlling stimulus is the binding of ligand molecules to intracellular or extracellular binding sites of the channel protein. The aim of this module is to offer an insight into different strategies on watching the ligand-gated ion channels at work. A standard approach to characterise their activation behaviour in response to a ligand stimulus is the electrophysiological patch-clamp technique. As a heterologous expression system we use the oocytes of the crall frog Xenopus laevis and cultured human embronic kidney cells (HEK cells). In the first part of our course the students will learn how to prepare these cells for the electrophysiological use. Furthermore they will get the opportunity to be introduced in different configurations of the patch-clamp technique, focussing on the inside-out and the outside-out configuration. To study not only the activation behaviour but also simultaneously the ligand binding, our group established the confocal patch-clamp fluorometry (confocal PCF). In this method we combine electrophysiological techniques and confocal fluorescence microscopy using fluorescently labelled ligand molecules. In the second part of the course the students will get the chance to make not only activation behaviour but also ligand binding visible. At the end, the collected electrophysiological and optical data will be analyzed using different analyzing and mathematical software.
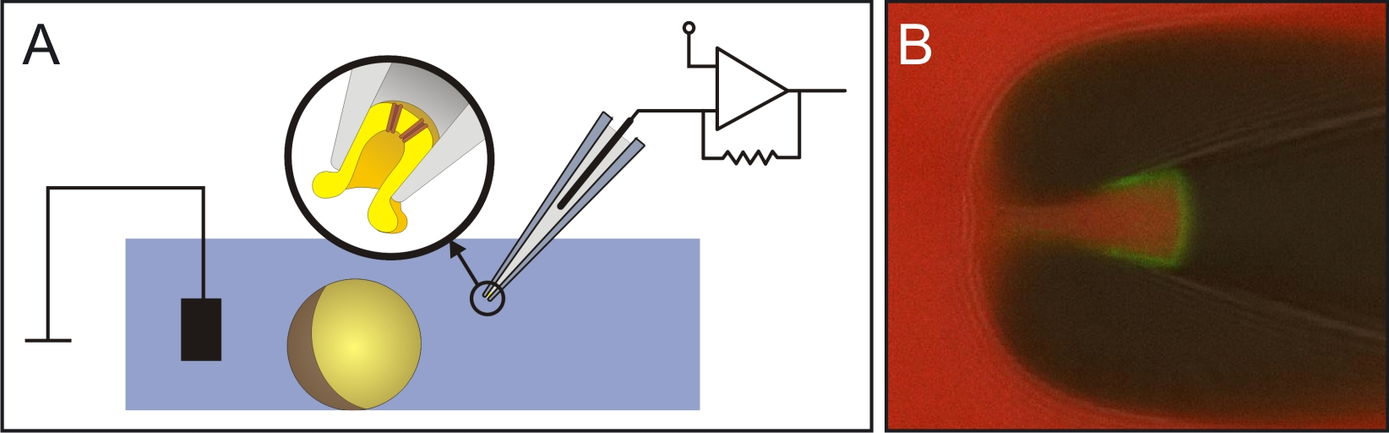
A) Scheme of a patch-clamp setup. The illustration shows the inside-out configuration with a glass pipette containing a membran patch excised from a Xenopus laevis oocyte.
B) Confocal image of a patch-pipette with an excised membrane patch expressing a high density of CNGA2 channels. The green fluorescence signal is due to the binding of fluroescently labelled cGMP molecules to the ion channels’ binding sites.

