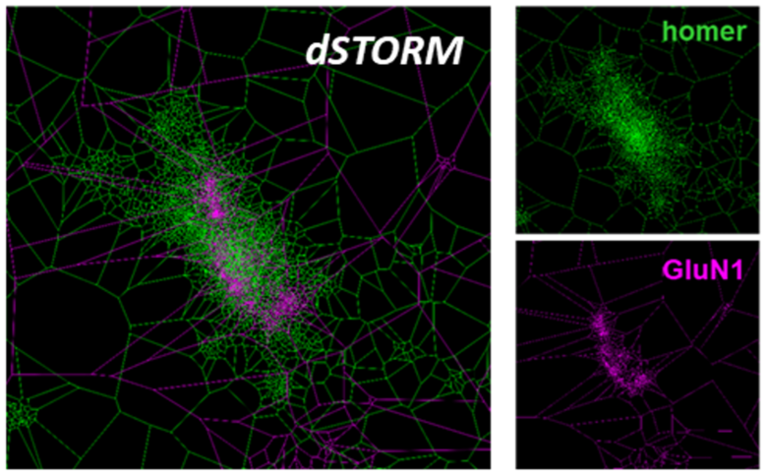
„Binding of human antibodies from a patient with NMDA receptor encephalitis to the GluN1 subunit
of the NMDA receptors as shown by super-resolution dSTORM imaging and tesselation cluster
analysis
Upper right picture: Homer staining; homer protein clusters and colocalize with NMDA receptors
right picture below: Same synapse, GluN1 staining
Left picture: overlay of homer and GluN1 staining

Section of Translational Neuroimmunology
The research interests of our group focusses on autoimmune mechanisms on central synaptic pathology.
Recent findings in patients with autoimmune encephalitis showed that these disorders are associated and presumably induced by highly specific autoantibodies against synaptic target structures, e.g. ionotropic receptors (NMDA, AMPA, Glycin receptors), G-protein coupled receptors (e.g. GABA-B receptors), or synaptic linker proteins (e.g. LGI1). The group of C. Geis investigates the effects of human autoantibodies on pre- and postsynaptic mechanisms. We have established passive-transfer mouse models with application of purified patient antibodies that are used to study the pathogenic effects of antibodies on animal behavior. Moreover, autoantibody-induced synaptic dysfunctions are evaluated in neuronal cell cultures, acute brain slices, and in-vivo using neurophysiological techniques. Super-resolution imaging techniques are used to investigate distinct antibody-induced morphological changes on the nanoscale level.
Another research focus is on pathomechanisms involved in sepsis associated encephalopathy. Here, the group of C. Geis uses a murine peritoneal contamination and infection model (PCI) to evaluate immune-mediated processes that may cause neuronal damage underlying cognitive deficits. Of particular interest is the role of microglia as the brain’s resident immune cells. Techniques used involve qPCR and RNASeq to analyze changes on the transcriptome level as well as immunohistochemical stainings of brain slices for morphological analyses. Several behavioural tests are performed to evaluate neurocognitive deficits after PCI.