Prof. Dr. Ignacio Rubio
Department of Anesthesiology and Intensive Care Medicine


Sepsis represents a lethal syndrome caused by a dysregulated und disproportionate immune reaction to infection. The intricacies of the sepsis–associated host immune response and the often concomitant multiple organ failure (MOF) underlie high mortality (sepsis is the major cause of death in ICUs!) and the extremely limited number of treatment options. In the acute phase of sepsis, the host response often manifests as a hyper-inflammatory burst (also known as “cytokine storm”) involving the profuse release of pro-inflammatory mediators. This can cause hemodynamic complications leading to endothelial barrier dysfunction all of which can further boost immune alterations, eventually culminating in organ failure and death.
In our laboratory at the Department of Anaesthesiology and Intensive Care Medicine, we investigate the abnormalities in signal transduction responses caused by and causative for sepsis in both immune and parenchymal stromal cells. We use different approaches, e.g., in vitro model systems for organ damage or the analysis of genetic paediatric syndromes that influence the host immune and inflammatory response. In this practical course, you will analyse the biological consequences of rare pathological variants of a critical regulator of Toll receptor and inflammasome signalling discovered in rare cases of paediatric encephalopathy. We will use a combination of biochemical and flow cytometric approaches and work with genetically modified cell lines that mimic the cellular phenotypes of patients. These experiments will give you an insight into how the mechanisms of a host immune response can be studied in the laboratory using state-of-the-art technologies.


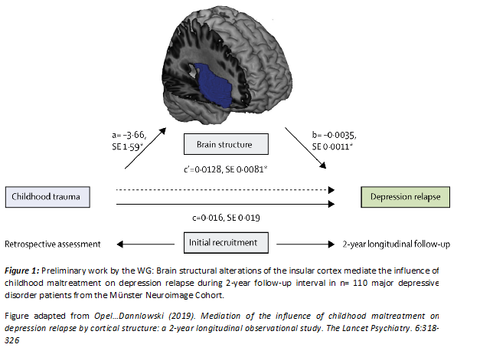
Psychiatric disorders such as major depression are among the most common disorders and represent one of the leading causes of disability worldwide. Maladaptive alterations in structure and function of neural circuits are increasingly recognized as a key mediator in the association between risk factors such as environmental stress and the development of psychiatric disorders. Despite the increasing knowledge on the neurobiological basis of psychiatric disorders, we are currently unable to estimate how successful any one course of action will be at alleviating symptoms for each individual patient. Against this backdrop, we aim (i) to generate insights on the risk factors and neurobiological mechanisms that determine individual disease trajectories with a special emphasis on affective disorders and (ii) to transfer these insights into optimized diagnostic, treatment and prevention in clinical routine. To this end, we conduct studies that employ a combination of multimodal neuroimaging (structural T1, rs-fMRI and DTI) and digitized longitudinal phenotyping in patients with affective disorders before and after different interventions (neurostimulation, pharmacology, psychotherapy). These investigations will allow to (a) disentangle neural circuit alterations in high-risk subgroups, (b) identify their value for an individual prediction of treatment response, (c) understand how interventions normalize maladaptive neural circuits to achieve remission.
During the summer school participants will be introduced to ongoing neuroimaging studies of the working group. They will thus gain insights on MR-measurements, clinical interviews, neuropsychological testing and digital phenotyping with patients. Motivated participants have the chance to work on a dedicated research question and analyze neuroimaging data under supervision of a team member.
