Diversity of transport mechanisms in the SLC1 transporter family
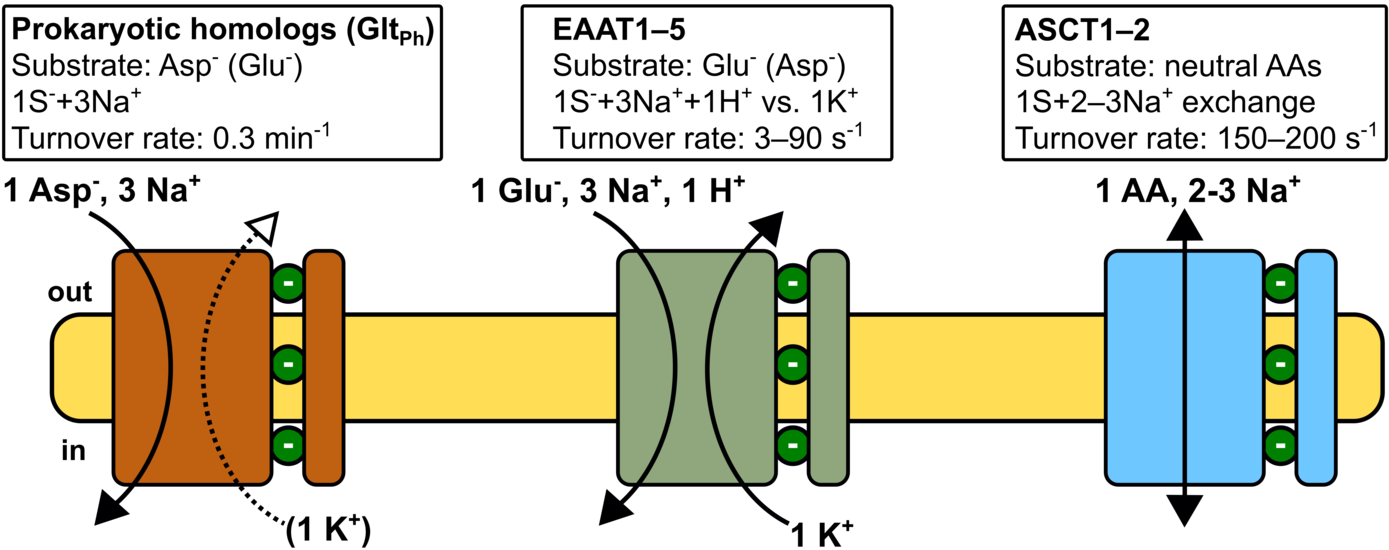
SLC1 transporters exhibit an impressive functional diversity ranging from Na+/K+/H+-coupled secondary active glutamate transport with complex transport stoichiometry in the mammalian EAATs, Na+-coupled aspartate uptake by prokaryotic homologues (e.g. GltPh or GltTk), to amino acid exchange in the neutral amino acid exchangers (ASCTs). We will combine atomistic molecular dynamics simulations, patch-clamp electrophysiology and fluorescence spectroscopy to investigate isoform-specific differences in Na+- and amino acid-binding kinetics and differences in ion-substrate coupling mechanisms. SLC1 proteins are prototypic elevator transporters, whereby substrate transport is accomplished via large-scale rigid-body movements of the transport domain across the membrane. Recent experimental structures of both GltPh and human ASCT2 define the start and endpoints of this shared transport mechanism. To establish the molecular principles underlying the adaptation of transport rates in the different isoforms, we will quantitatively investigate the energetics and kinetics of transmembrane translocation and the functional implications of protein-lipid interactions in these isoforms. Finally, glutamate transporters are potentially important - yet clinically unused - pharmacological targets to mitigate glutamate excitotoxicity in various brain diseases. Recently, several positive allosteric modulators were identified, which increase EAAT transport activity and exhibit neuroprotective effects. We will investigate how such compounds modulate glutamate translocation using molecular simulations, cryo-EM, and electrophysiology to establish a mechanistic framework for allosteric EAAT activators.
Prof. Dr. Christoph Fahlke
52425 Jülich
Jun-Prof. Dr. Jan-Philipp Machtens
52425 Jülich

