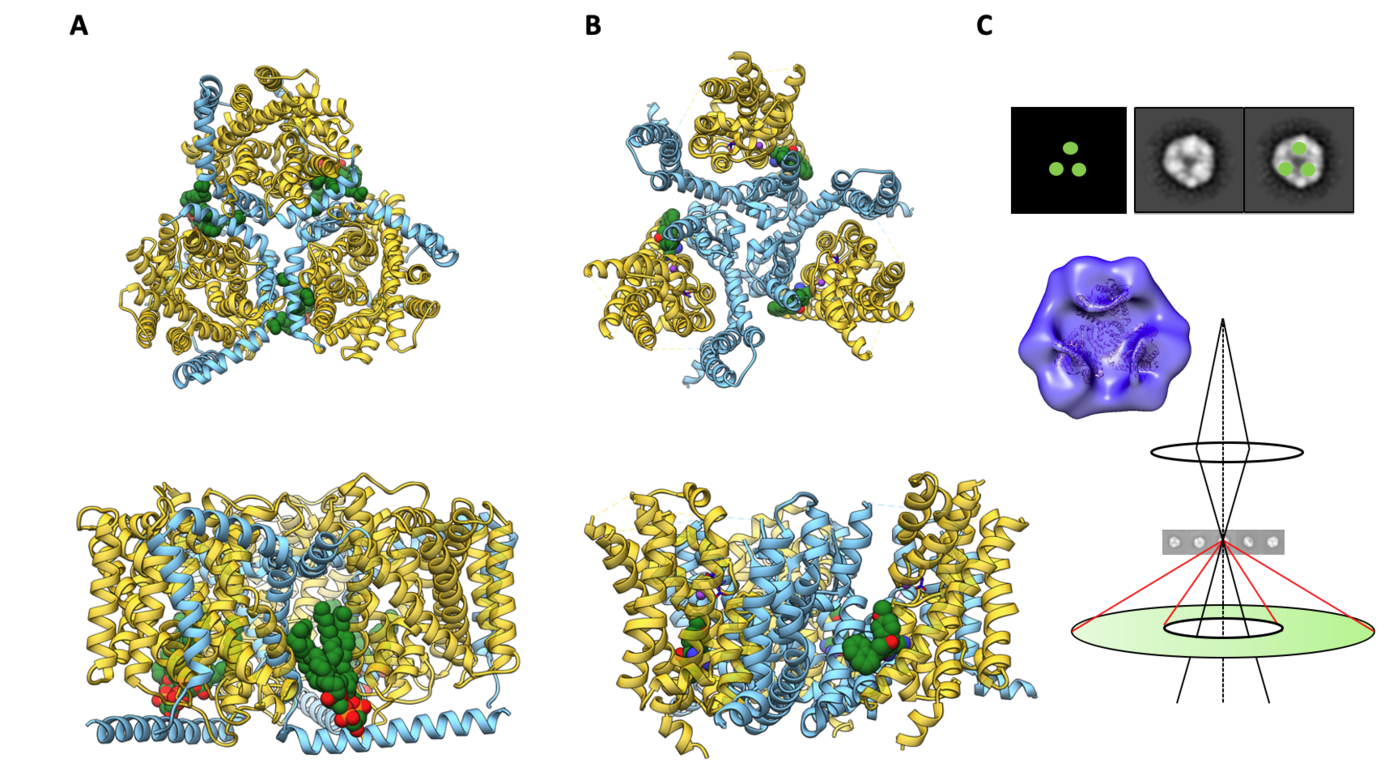
Role of lipids on the conformational cycling in trimeric Na+-coupled symporters
The secondary transporter function is highly affected by oligomerization. One prime example is the Na+ coupled betaine transporter BetP, a member of the BCCT family, which is regulated exclusively as a trimer. In trimeric state, specific lipid contacts as well as intra-trimeric interactions of terminal domains take place resulting in a re-orientation of individual protomers with respect to the membrane normal. As a consequence, the turn-over rate and cooperativity of Na+ coupling in BetP is altered in a K+ dependent manner. Being structurally unrelated, Na+/K+/H+-coupled glutamate transporters of the SLC1 family, e.g. EAAT1, share the trimeric assembly with BetP. The central ‘trimerization domain’ in SLC1 transporters deeply embedded in the membrane enables the characteristic and independent elevator movement of the three ‘transport domains’ during alternating access. In BetP, the C-terminal domain is the major transport regulator. Likewise, in EAAT1 and EAAT2, the respective C-terminal domains were shown to modify the glutamate uptake cycle and chloride permeability.
In this project, we want to investigate if there are common mechanistic pattern in trimeric EAAT1 and BetP, by which regulatory and/or modulating lipid interactions affect the cooperativity of Na+ coupling and K+ antiport. Any mechanistic similarities between these evolutionary unrelated trimeric symporters might suggest very general principles in sodium-coupled transport regulation. Therefore, we want to perform a comparative structure-dynamics study between BetP and EAAT1. We want to investigate (1) the role of trimerization as interaction platform for lipids and modulators, and (2) the regulatory role of K+ on cooperative sodium coupling in BetP and EAAT1. To achieve this goal, we want to use these trimeric transporters, for which extensive structural information is already available to establish new tools in structural biology using mainly cryo scanning transmission electron microscopy (cryo-STEM) and single molecule scanning near-field optical microscopy (s-SNOM) in FTIR mode. By the means of cryo-STEM we want to assign K+ (Rb+) densities in cryo-EM structures to determine K+ coordination in BetP and EAAT1 in native lipid environment. To investigate dynamic regulatory lipid interactions exploiting the trimeric architecture of the transporters, we will rely on the innovative scanning-based spectroscopy method s-SNOM in an FTIR mode.